Contents
Document history
The controlled copy of this document is maintained in the Genomics England internal document management system. Any copies of this document held outside that system, in whatever format (for example, paper, email attachment), are considered to have passed out of control and should be checked for currency and validity. This document is uncontrolled when printed.
About this document
This document provides a summary of the Publication Moratorium that affords certain GeCIP domains preferential permission to work on data in their area of research. The proposed system makes assumptions about the schedule and system for data release into the Research Environment, which may turn out to be different; suggestions and comments are welcome. The moratorium summary will ultimately form part of the GeCIP guidance for research and publication document.
Moratorium summary
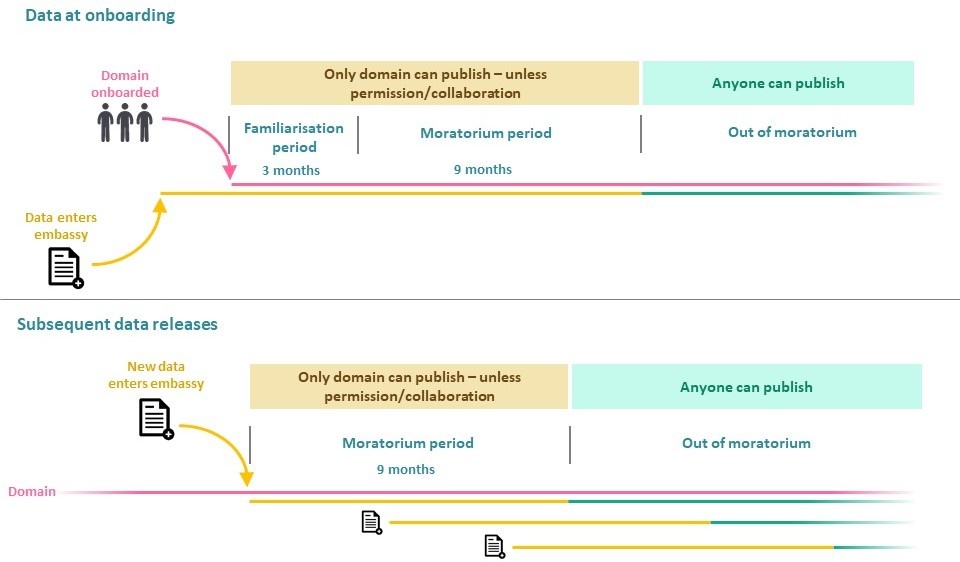
Genomics England has instigated a per participant Publication Moratorium on data of all types (including that generated from clinical reports) from the 100,000 Genomes Project, offering a degree of preferential permission to analyse participant data for publication, in recognition of those domains and individuals that have assisted in recruiting to the project. In effect, the moratorium will prevent other GeCIP researchers from carrying out research on that domain’s corresponding data until the domain has had access to the data for at least nine months. The moratorium can be disregarded on a case by case basis for a given project if there is prior agreement from the domain’s lead or there is collaboration between the respective domains.
It is expected that individual domains will not be unduly obstructive in requests for collaboration, and will consider cross-domain collaboration over competition, where they feel there is conflict with their research plans. The Publication Moratorium does not prevent any individual accessing any data, merely restricts the analysis for publication of any findings from a domain’s corresponding data to members of another domain.
The moratorium period applies individually to data from each participant, starting once data have been released into the Research Environment. Individuals who recruit participants into the Project can therefore be assured that regardless of the point at which they recruit a participant to the Project, the participant’s data will be protected under the moratorium on its entry into the Research Environment (although this is independent of whether that individual may have access to the Research Environment or not).
After the moratorium ends
Once the moratorium period has passed for a participant, any researcher may analyse this participant’s data, although it is expected that collaborations will be pursued between domains and researchers so that the research project benefits from the extensive expertise present within the GeCIP community. Out of moratorium, publications will undergo a mandatory assessment by Genomics England on a ‘first-come, first-served’ basis independent of when or by whom the original research project was registered. This process will check whether any publication moratorium has been broken, that no identifiable data has been inadvertently released, whether the paper contains any intellectual property that may need to be protected, and that the paper has been prepared in accordance with the stipulations made in the Publication Policy.
Registering research
All research projects expected to result in a publication must be registered in the Research Registry within the Research Environment. During the registration of a research project on the Research Registry, the research lead (the lead of the individual research project not necessarily the lead of the GeCIP domain) will be asked to select the disease or tumour types whose samples will form the core of their analysis (this need not include any control samples). Should any of the diseases or tumour types selected include samples that do not correspond to their domain membership, they will be reminded that they may need to enter into collaboration with the relevant domain to be able to use certain participants’ data.
How are data assigned to a GeCIP domain?
The assignment of samples and data to a domain is governed by the disease or tumour type under which the participant and their relatives were recruited to the Project. In the case of families recruited to the rare disease arm of the Project, the associated disorders are determined by the gene panels applied at the interpretation stage, to ensure that all the relevant disorders are captured. Recruited relatives of the proband may often be unaffected, but their data is nevertheless assigned to the disorder(s) under which the proband was recruited.
Some participants are recruited to the Project under more than one disease category and their data may therefore be associated with multiple GeCIP domains. In this case, the ‘owner’ domains may all work on this data and do not need to seek permission or collaboration from each other. Researchers in other domains who wish to analyse data from these participants need only seek permission or collaboration with one of the owner domains.
Accessing data
The onboarding of GeCIP domains will be staggered with the timing largely dictated by the relative abundance of samples and data corresponding to the domain. As and when Genomics England feels there are sufficient data for the domain to begin analyses, available researchers (ie those verified by an institution that has signed the Participation Agreement) will be granted access. Upon onboarding of a domain, the moratorium period will begin immediately for the domain’s participant data already present within the Research Environment, comprising a three-month familiarisation period followed by the nine-month Publication Moratorium period. The familiarisation period is a one-time event that applies only for the three months after the onboarding of the domain to allow them to learn how to use the Research Environment and access data. Prior to onboarding of a domain, Genomics England will regularly circulate to the domain details of projects registered by onboarded researchers and assist in any collaboration requests.
Data releases
Following initial onboarding of GeCIP domains, de-identified participant data will enter the Research Environment in regular quarterly batches consisting of all participants to date for which clinical reports have been generated; thus, each version of the research dataset will represent the most up-to-date version of the most complete portion of the dataset. Participant data released to the Research Environment will include associated values showing the date of entry to the environment, the date from which this participant’s data are no longer under moratorium and the associated domain(s) to which the data belongs. LabKey views within the Research Environment will enable researchers to easily select cohorts by disease or tumour type and moratorium status. The start of the Publication Moratorium for a given participant’s data will be taken as the first instance in which their data was released into the Research Environment (or the date three months following the relevant domain being onboarded to the environment if the data was present prior to domain onboarding).
Which domains are protected by the moratorium?
Only Rare Disease and Cancer (excluding Pan Cancer) GeCIP domains will be granted protection by the Publication Moratorium. Collaboration or obtaining consent is encouraged in general regardless of the moratorium status of samples. While GeCIP has been structured to minimise overlap in research interest and focus between the domains (and the Access Review Committee approval process enforces this), this cannot be removed entirely. It is hoped that researchers will, through cooperation and collaboration, realise the benefit of a community with broad experience and interests, and actively avoid any redundancy in research efforts. The GeCIP Board and/or the Publication Committee will act on any suggestion that a researcher or domain is being unduly obstructive.